Modern Theory Of The Atom
3.ane Diminutive Theory
Learning Objectives
- State the modern atomic theory.
- Learn how atoms are constructed.
The smallest piece of an chemical element that maintains the identity of that element is called an atomThe smallest piece of an chemical element that maintains the identity of that element. . Private atoms are extremely small-scale. It would accept about fifty million atoms in a row to brand a line that is i cm long. The period at the end of a printed sentence has several one thousand thousand atoms in information technology. Atoms are so modest that information technology is difficult to believe that all thing is fabricated from atoms—simply information technology is.
The concept that atoms play a key function in chemistry is formalized by the mod atomic theoryThe concept that atoms play a fundamental role in chemistry. , first stated by John Dalton, an English language scientist, in 1808. Information technology consists of 3 parts:
- All matter is equanimous of atoms.
- Atoms of the same element are the same; atoms of unlike elements are different.
- Atoms combine in whole-number ratios to form compounds.
These concepts form the basis of chemistry.
Although the word atom comes from a Greek word that means "indivisible," nosotros understand now that atoms themselves are composed of smaller parts chosen subatomic particles. The first part to be discovered was the electronA tiny subatomic particle with a negative charge. , a tiny subatomic particle with a negative accuse. It is often represented as e−, with the right superscript showing the negative charge. Later, two larger particles were discovered. The protonA subatomic particle with a positive accuse. is a more massive (just still tiny) subatomic particle with a positive charge, represented equally p+. The neutronA subatomic particle with no charge. is a subatomic particle with about the same mass as a proton simply no charge. Information technology is represented as either n or north0. Nosotros now know that all atoms of all elements are composed of electrons, protons, and (with i exception) neutrons. Table 3.i "Properties of the Three Subatomic Particles" summarizes the backdrop of these three subatomic particles.
Table iii.1 Properties of the Three Subatomic Particles
| Name | Symbol | Mass (approx.; kg) | Charge |
|---|---|---|---|
| Proton | p+ | one.6 × 10−27 | 1+ |
| Neutron | n, n0 | 1.6 × 10−27 | none |
| Electron | e− | 9.1 × ten−31 | ane− |
How are these particles arranged in atoms? They are non bundled at random. Experiments by Ernest Rutherford in England in the 1910s pointed to a nuclear modelThe model of an atom that has the protons and neutrons in a fundamental nucleus with the electrons in orbit near the nucleus. of the atom. The relatively massive protons and neutrons are nerveless in the center of an atom, in a region called the nucleusThe center of an atom that contains protons and neutrons. of the atom (plural nuclei). The electrons are outside the nucleus and spend their time orbiting in space nearly the nucleus. (Run into Figure 3.1 "The Construction of the Atom".)
Figure three.1 The Structure of the Atom
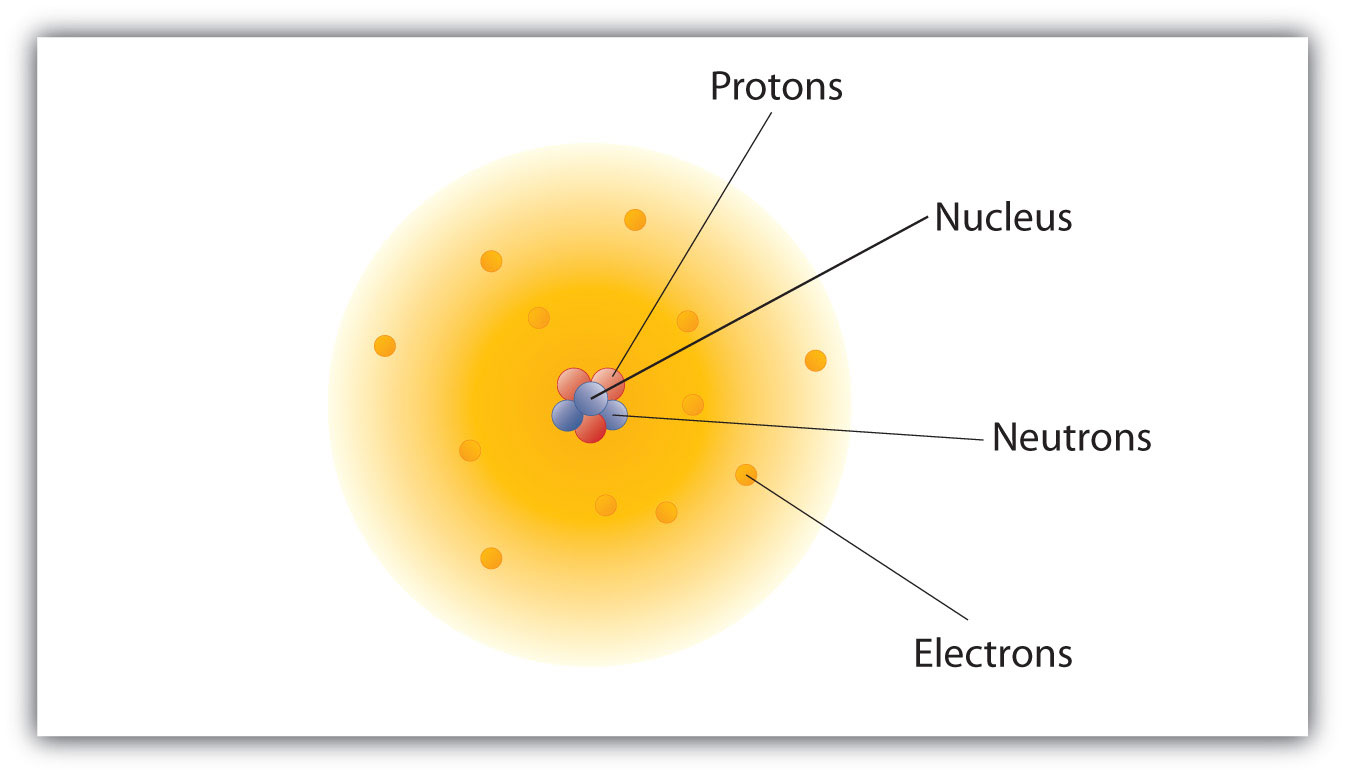
Atoms take protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus.
The modern atomic theory states that atoms of 1 element are the same, while atoms of dissimilar elements are different. What makes atoms of different elements dissimilar? The central feature that all atoms of the aforementioned chemical element share is the number of protons. All atoms of hydrogen have one and only one proton in the nucleus; all atoms of iron have 26 protons in the nucleus. This number of protons is and then important to the identity of an atom that it is chosen the atomic numberThe number of protons in an cantlet. of the element. Thus, hydrogen has an diminutive number of one, while iron has an atomic number of 26. Each element has its own characteristic atomic number.
Atoms of the same element tin have different numbers of neutrons, however. Atoms of the same element (i.e., atoms with the aforementioned number of protons) with unlike numbers of neutrons are called isotopesAtoms of the same element that have different numbers of neutrons. . Nigh naturally occurring elements be every bit isotopes. For case, most hydrogen atoms have a single proton in their nucleus. All the same, a pocket-size number (near one in a million) of hydrogen atoms take a proton and a neutron in their nuclei. This particular isotope of hydrogen is called deuterium. A very rare grade of hydrogen has one proton and two neutrons in the nucleus; this isotope of hydrogen is chosen tritium. The sum of the number of protons and neutrons in the nucleus is chosen the mass numberThe sum of the number of protons and neutrons in a nucleus. of the isotope.
Neutral atoms have the aforementioned number of electrons equally they have protons, then their overall charge is zero. However, every bit we shall come across later, this will not always be the case.
Example ane
- The most common carbon atoms have six protons and six neutrons in their nuclei. What are the atomic number and the mass number of these carbon atoms?
- An isotope of uranium has an diminutive number of 92 and a mass number of 235. What are the number of protons and neutrons in the nucleus of this atom?
Solution
- If a carbon atom has six protons in its nucleus, its diminutive number is half dozen. If information technology besides has six neutrons in the nucleus, so the mass number is half dozen + half dozen, or 12.
- If the atomic number of uranium is 92, so that is the number of protons in the nucleus. Because the mass number is 235, and then the number of neutrons in the nucleus is 235 − 92, or 143.
Test Yourself
The number of protons in the nucleus of a tin atom is fifty, while the number of neutrons in the nucleus is 68. What are the atomic number and the mass number of this isotope?
Answer
Atomic number = fifty, mass number = 118
When referring to an atom, we simply use the element's proper noun: the term sodium refers to the element as well equally an cantlet of sodium. But it tin be unwieldy to use the name of elements all the time. Instead, chemistry defines a symbol for each chemical element. The atomic symbolA ane- or two-alphabetic character representation of the proper name of an element. is a one- or two-alphabetic character abridgement of the name of the element. By convention, the first letter of the alphabet of an chemical element's symbol is always capitalized, while the second letter (if present) is lowercase. Thus, the symbol for hydrogen is H, the symbol for sodium is Na, and the symbol for nickel is Ni. Most symbols come from the English name of the element, although some symbols come from an chemical element'southward Latin name. (The symbol for sodium, Na, comes from its Latin name, natrium.) Table 3.2 "Names and Symbols of Mutual Elements" lists some common elements and their symbols. You should memorize the symbols in Tabular array 3.ii "Names and Symbols of Common Elements", as this is how we will be representing elements throughout chemistry.
Table three.ii Names and Symbols of Common Elements
| Element Name | Symbol | Element Name | Symbol | |
|---|---|---|---|---|
| Aluminum | Al | Mercury | Hg | |
| Argon | Ar | Molybdenum | Mo | |
| Arsenic | As | Neon | Ne | |
| Barium | Ba | Nickel | Ni | |
| Glucinium | Be | Nitrogen | N | |
| Bismuth | Bi | Oxygen | O | |
| Boron | B | Palladium | Pd | |
| Bromine | Br | Phosphorus | P | |
| Calcium | Ca | Platinum | Pt | |
| Carbon | C | Potassium | One thousand | |
| Chlorine | Cl | Radium | Ra | |
| Chromium | Cr | Radon | Rn | |
| Cobalt | Co | Rubidium | Rb | |
| Copper | Cu | Scandium | Sc | |
| Fluorine | F | Selenium | Se | |
| Gallium | Ga | Silicon | Si | |
| Germanium | Ge | Silver | Ag | |
| Gold | Au | Sodium | Na | |
| Helium | He | Strontium | Sr | |
| Hydrogen | H | Sulfur | Southward | |
| Iodine | I | Tantalum | Ta | |
| Iridium | Ir | Can | Sn | |
| Fe | Fe | Titanium | Ti | |
| Krypton | Kr | Tungsten | Due west | |
| Pb | Pb | Uranium | U | |
| Lithium | Li | Xenon | Xe | |
| Magnesium | Mg | Zinc | Zn | |
| Manganese | Mn | Zirconium | Zr |
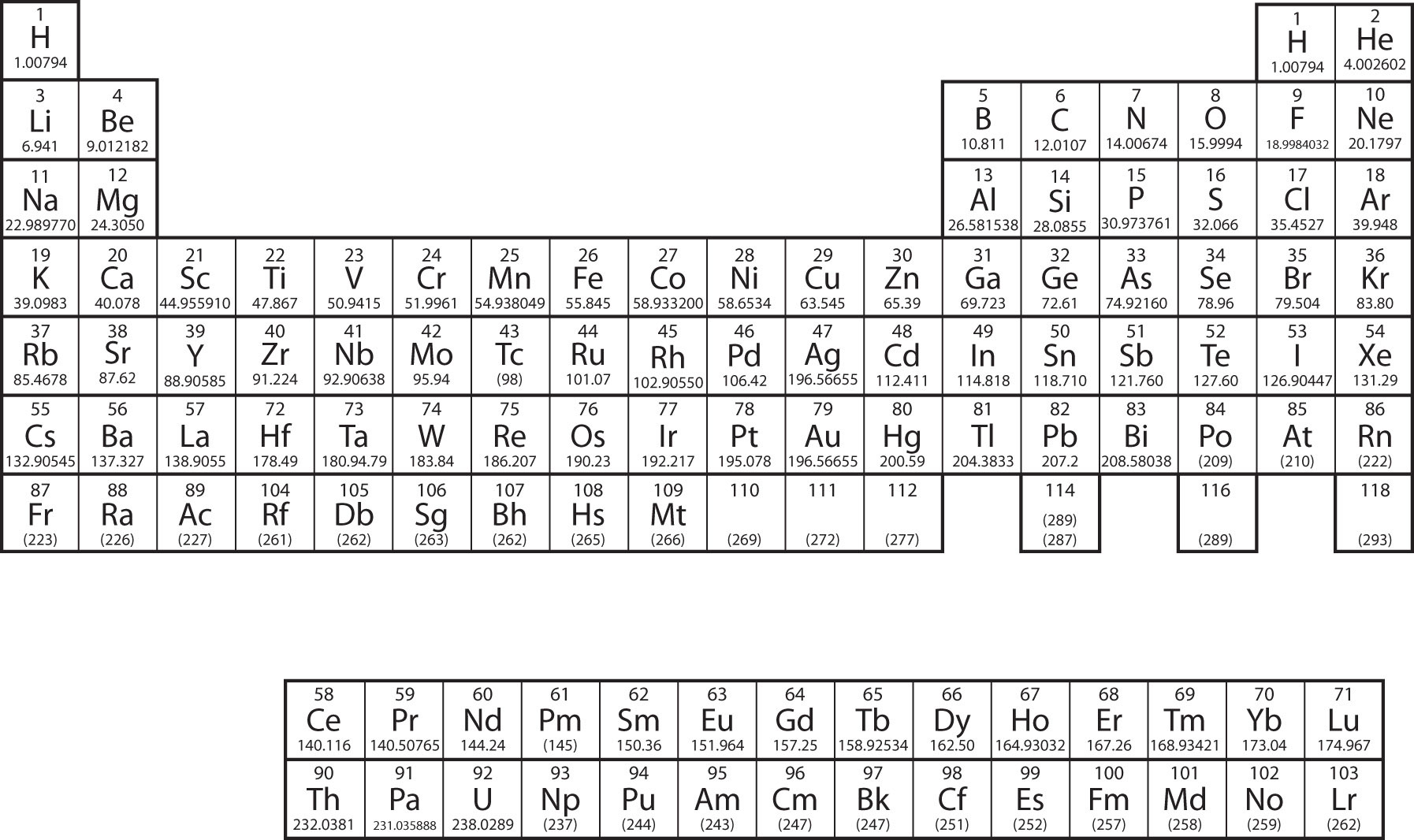
The elements are grouped together in a special chart called the periodic tabular arrayA chart of all the elements. . A uncomplicated periodic table is shown in Figure 3.ii "A Uncomplicated Periodic Table", while a more extensive one is presented in Chapter 17 "Appendix: Periodic Table of the Elements". The elements on the periodic table are listed in club of ascending atomic number. The periodic table has a special shape that will become important to the states when we consider the organization of electrons in atoms (see Affiliate viii "Electronic Structure"). One immediate use of the periodic table helps u.s.a. place metals and nonmetals. Nonmetals are in the upper right corner of the periodic table, on i side of the heavy line splitting the right-hand part of the chart. All other elements are metals.
Effigy three.2 A Simple Periodic Table
At that place is an like shooting fish in a barrel way to stand for isotopes using the atomic symbols. Nosotros use the construction
where 10 is the symbol of the chemical element, A is the mass number, and Z is the atomic number. Thus, for the isotope of carbon that has 6 protons and vi neutrons, the symbol is
where C is the symbol for the element, 6 represents the atomic number, and 12 represents the mass number.
Case 2
- What is the symbol for an isotope of uranium that has an atomic number of 92 and a mass number of 235?
- How many protons and neutrons are in ?
Solution
- The symbol for this isotope is .
- This iron atom has 26 protons and 56 − 26 = 30 neutrons.
Test Yourself
How many protons are in ?
Answer
xi protons
It is also mutual to state the mass number subsequently the name of an element to indicate a detail isotope. Carbon-12 represents an isotope of carbon with half dozen protons and 6 neutrons, while uranium-238 is an isotope of uranium that has 146 neutrons.
Key Takeaways
- Chemistry is based on the modernistic atomic theory, which states that all thing is equanimous of atoms.
- Atoms themselves are composed of protons, neutrons, and electrons.
- Each element has its own diminutive number, which is equal to the number of protons in its nucleus.
- Isotopes of an element comprise different numbers of neutrons.
- Elements are represented by an atomic symbol.
- The periodic tabular array is a nautical chart that organizes all the elements.
Exercises
-
List the three statements that brand upwardly the modern atomic theory.
-
Explicate how atoms are composed.
-
Which is larger, a proton or an electron?
-
Which is larger, a neutron or an electron?
-
What are the charges for each of the iii subatomic particles?
-
Where is most of the mass of an atom located?
-
Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus.
-
Sketch a diagram of a helium atom, which has two protons and two neutrons in its nucleus.
-
Define atomic number. What is the diminutive number for a boron atom?
-
What is the atomic number of helium?
-
Define isotope and give an example.
-
What is the difference betwixt deuterium and tritium?
-
Which pair represents isotopes?
- and
- and
- and
-
Which pair represents isotopes?
- and
- and
- and
-
Give complete symbols of each atom, including the diminutive number and the mass number.
- an oxygen atom with 8 protons and 8 neutrons
- a potassium atom with nineteen protons and twenty neutrons
- a lithium atom with 3 protons and 4 neutrons
-
Give complete symbols of each atom, including the atomic number and the mass number.
- a magnesium atom with 12 protons and 12 neutrons
- a magnesium atom with 12 protons and 13 neutrons
- a xenon atom with 54 protons and 77 neutrons
-
Americium-241 is an isotope used in smoke detectors. What is the complete symbol for this isotope?
-
Carbon-14 is an isotope used to perform radioactive dating tests on previously living material. What is the consummate symbol for this isotope?
-
Give atomic symbols for each element.
- sodium
- argon
- nitrogen
- radon
-
Requite atomic symbols for each element.
- silver
- aureate
- mercury
- iodine
-
Requite the name of the element.
- Si
- Mn
- Fe
- Cr
-
Give the name of the element.
- F
- Cl
- Br
- I
Answers
-
All thing is composed of atoms; atoms of the aforementioned chemical element are the same, and atoms of unlike elements are unlike; atoms combine in whole-number ratios to class compounds.
-
A proton is larger than an electron.
-
proton: 1+; electron: 1−; neutron: 0
-
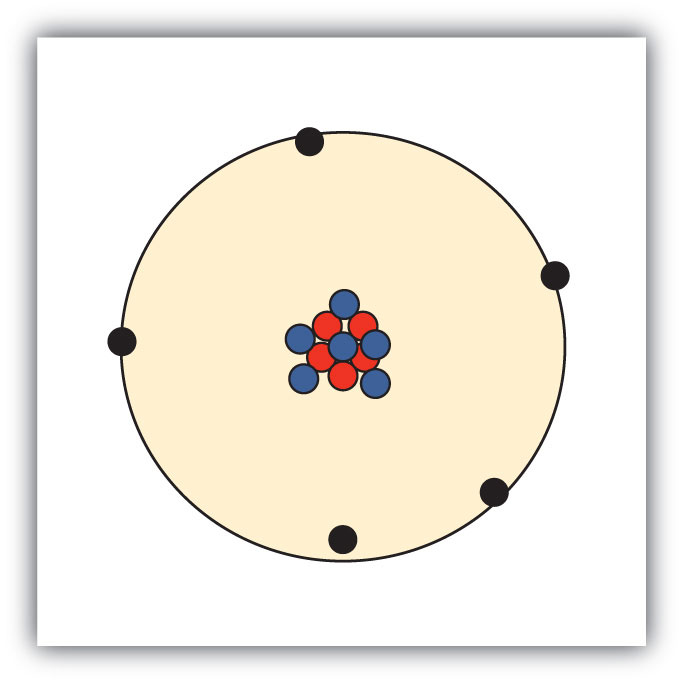
-
The atomic number is the number of protons in a nucleus. Boron has an atomic number of five.
-
Isotopes are atoms of the aforementioned element just with different numbers of neutrons. and are examples.
-
- isotopes
- not isotopes
- not isotopes
-
-
-
- Na
- Ar
- Northward
- Rn
-
- silicon
- manganese
- atomic number 26
- chromium
Modern Theory Of The Atom,
Source: https://saylordotorg.github.io/text_introductory-chemistry/s07-01-atomic-theory.html
Posted by: greenewheyes.blogspot.com


0 Response to "Modern Theory Of The Atom"
Post a Comment